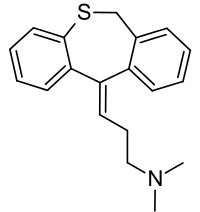
Dosulepin
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Dothep, Prothiaden |
| AHFS/Drugs.com | International Drug Names |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | N06AA16 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 30%[1] |
| Protein binding | 84%[2] |
| Metabolism | Hepatic (via N-demethylation to active metabolite northiaden, S-oxidation and glucuronidation)[2] |
| Biological half-life | 51 hours,[2] 14-45 hours,[3][4][5] 54 hours (elderly)[5][6] |
| Excretion | Urine (56%), faeces (15%)[2] |
| Identifiers | |
| |
| CAS Number |
113-53-1 |
| PubChem (CID) | 13473 |
| ChemSpider |
4447605 |
| UNII |
W13O82Z7HL |
| KEGG |
D07872 |
| ChEMBL |
CHEMBL108947 |
| ECHA InfoCard | 100.003.665 |
| Chemical and physical data | |
| Formula | C19H21NS |
| Molar mass | 295.45 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Dosulepin (INN, BAN) formerly known as dothiepin (USAN), and marketed under the brand names Prothiaden, Dothep, Thaden, and Dopress, is a tricyclic antidepressant that is used in several European and South Asian countries, as well as Australia, South Africa, and New Zealand. It is not used in the United States.[7]
Medical uses
Dosulepin is used for the treatment of major depressive disorder and neuropathic pain.[1] Dosulepin is only TGA- and MHRA-approved for the treatment of major depressive disorder.[8][9] There is clear evidence of the efficacy of dosulepin in psychogenic facial pain, though the drug may be needed for up to a year.[10]
Adverse effects
Common adverse effects:[2]
- Drowsiness
- Extrapyramidal symptoms
- Tremor
- Confusion
- Disorientation
- Dizziness
- Paresthesias
- Alterations to ECG patterns
- Dry mouth
- Sweating
- Urinary retention
- Hypotension
- Postural hypotension
- Tachycardia
- Palpitations
- Arrhythmias
- Conduction defects
- Increased or decreased libido
- Nausea
- Vomiting
- Constipation
- Blurred vision
Less common adverse effects:[2]
- Disturbed concentration
- Delusions
- Hallucinations
- Anxiety
- Fatigue
- Headaches
- Restlessness
- Excitement
- Insomnia
- Hypomania
- Nightmares
- Peripheral neuropathy
- Ataxia
- Incoordination
- Seizures
- Paralytic ileus
- Hypertension
- Heart block
- Myocardial infarction
- Stroke
- Gynecomastia (swelling of breast tissue in males)
- Testicular swelling
- Impotence
- Epigastric distress
- Abdominal cramps
- Parotid swellings
- Diarrhea
- Stomatitis (swelling of the mouth)
- Black tongue
- Peculiar taste sensations
- Cholestatic jaundice
- Altered liver function
- Hepatitis (swelling of the liver)
- Skin rash
- Urticaria (hives)
- Photosensitisation
- Skin blisters
- Angioneurotic edema
- Weight loss
- Urinary frequency
- Mydriasis
- Weight gain
- Hyponatremia (low blood sodium)
- Movement disorders
- Dyspepsia (indigestion)
- Increased intraocular pressure
- Changes in blood sugar levels
- Thrombocytopenia (an abnormally low number of platelets in the blood. This makes one more susceptible to bleeds)
- Eosinophilia (an abnormally high amount of eosinophils in the blood)
- Agranulocytosis (a dangerously low number of white blood cells in the blood leaving one open to potentially life-threatening infections)
- Galactorrhea (lactation that is unassociated with breastfeeding and lactation)
Contraindications
Contraindications include:[2]
- Epilepsy as it can lower the seizure threshold
- TCAs should not be used concomitantly or within 14 days of treatment with monoamine oxidase inhibitors due to the risk for serotonin syndrome
- Acute recovery phase following myocardial infarction as TCAs may produce conduction defects and arrhythmias
- Liver failure
- Hypersensitivity to dothiepin
Drug interactions
Dosulepin can potentiate the effects of alcohol and at least one death has been attributed to this combination.[2] TCAs potentiate the sedative effects of barbiturates, tranquillisers and CNS depressants.[2] Guanethidine and other adrenergic neurone blocking drugs can have their antihypertensive effects blocked by dosulepin.[2] Sympathomimetics may potentiate the sympathomimetic effects of dosulepin.[2] Due to the anticholinergic and antihistamine effects of dosulepin anticholinergic and antihistamine medications may have their effects potentiated by dosulepin and hence these combinations are advised against.[2] Dosulepin may have its postural hypotensive effects potentiated by diuretics.[2] Anticonvulsants may have their efficacy reduced by dosulepin due to its ability to reduce the seizure threshold.[2]
Overdose
The symptoms and the treatment of an overdose are largely the same as for the other tricyclic antidepressants.[8] Dosulepin may be particularly toxic in overdose compared to other TCAs.[8] The onset of toxic effects is around 4–6 hours after dosulepin is ingested.[2] In order to minimise the risk of overdose it is advised that patients only receive a limited number of tablets at a time so as to limit their risk of overdosing.[2] It is also advised that patients are not prescribed any medications that are known to increase the risk of toxicity in those receiving dosulepin due to the potential for mixed overdoses.[2] The medication should also be kept out of reach of children.[2]
Mechanism of action
Dosulepin is a serotonin-norepinephrine reuptake inhibitor (SNRI) with anticholinergic, antihistamine, and antiadrenergic effects.[11]
| Receptor/Transporter | Ki (nM)[12] |
|---|---|
| SERT | 8.6 |
| NET | 46 |
| DAT | 5,310 |
| 5-HT1A | 4,004 |
| 5-HT2A | 152 |
| M1 | 18 |
| M2 | 109 |
| M3 | 38 |
| M4 | 61 |
| M5 | 92 |
| α1 | 419 |
| α2 | 12 |
| H1 | 4 |
Pharmacokinetics
Dothiepin is readily absorbed from the small intestine and is extensively metabolised on first-pass through the liver into its chief active metabolite, northiaden (desmethyldosulepin).[2] Peak plasma concentrations of between 30.4 ng/mL to 278.8 ng/mL occur within 2–3 hours of oral administration.[2] It is distributed in breast milk and crosses the placenta and blood-brain barrier.[2] It is highly bound to plasma proteins (84%), and has a whole-body elimination half-life of 51 hours.[2]
See also
References
- 1 2 Lancaster, SG; Gonzalez, JP (July 1989). "Dothiepin: a review of its pharmcodynamic and pharmacokinetic properties, and therapeutic efficacy in depressive illness". Drugs. 38 (1): 123–147. doi:10.2165/00003495-198938010-00005. PMID 2670509.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 "Dothep Dothiepin hydrochloride" (PDF). TGA eBusiness Services. Alphapharm Pty Limited. 1 November 2013. Retrieved 3 December 2013.
- ↑ Rees, JA (1981). "Clinical interpretation of pharmacokinetic data on dothiepin hydrochloride (Dosulepin, Prothiaden)". Journal of International Medical Research. 9 (2): 98–102. PMID 7227628.
- ↑ Maguire, KP; Burrows, GD; Norman, TR; Scoggins, BA (September 1981). "Metabolism and pharmacokinetics of dothiepin" (PDF). British Journal of Clinical Pharmacology. 12 (3): 405–409. doi:10.1111/j.1365-2125.1981.tb01235.x. PMC 1401810
 . PMID 7295471.
. PMID 7295471. - 1 2 Bareggi, SR; Cavallaro, R; Pirola, R; Altamura, AC (1990). "Pharmacokinetics and adverse effects of single doses of dothiepin in young and elderly subjects". Progress in Neuro-Psychopharmacology and Biological Psychiatry. 14 (2): 163–170. doi:10.1016/0278-5846(90)90098-2. PMID 2309034.
- ↑ Ogura, C; Kishimoto, A; Mizukawa, R; Hazama, H; Honma, H; Kawahara, K (1983). "Age differences in effects on blood pressure, flicker fusion frequency, salivation and pharmacokinetics of single oral doses of dothiepin and amitriptyline". European Journal of Clinical Pharmacology. 25 (6): 811–814. doi:10.1007/BF00542525. PMID 6662179.
- ↑ Dosulepin Hydrochloride. Martindale: The Complete Drug Reference. London, UK: Pharmaceutical Press. 5 December 2011. Retrieved 3 December 2013.
- 1 2 3 Rossi, S, ed. (2013). Australian Medicines Handbook (2013 ed.). Adelaide: The Australian Medicines Handbook Unit Trust. ISBN 978-0-9805790-9-3.
- ↑ Joint Formulary Committee (2013). British National Formulary (BNF) (65 ed.). London, UK: Pharmaceutical Press. ISBN 978-0-85711-084-8.
- ↑ C Feinmann; M Harris; R Cawley (11 February 1984). "Psychogenic facial pain: presentation and treatment.". Br Med J (Clin Res Ed). 288: 436–8. doi:10.1136/bmj.288.6415.436. PMC 1444752
 . PMID 6419955.
. PMID 6419955. - ↑ Brunton, L; Chabner, B; Knollman, B (2010). Goodman and Gilman's The Pharmacological Basis of Therapeutics (12th ed.). New York: McGraw-Hill Professional. ISBN 978-0-07-162442-8.
- ↑ Roth, BL; Driscol, J (12 January 2011). "PDSP Ki Database". Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. Retrieved 3 December 2013.