Bunazosin
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| ATC code | none |
| Identifiers | |
| |
| CAS Number | 80755-51-7 |
| PubChem (CID) | 2472 |
| ChemSpider |
2378 |
| UNII |
9UUW4V7G2H |
| ChEMBL |
CHEMBL188185 |
| Chemical and physical data | |
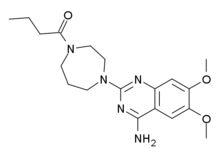
| Formula | C19H27N5O3 |
| Molar mass | 373.44 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| (verify) | |
Bunazosin (INN) is an alpha 1 antagonist. Bunazosin was initially developed to treat benign prostatic hyperplasia (BPH). It has been approved in Japan in a topical form to treat glaucoma. The mechanism of action is a reduction of aqueous outflow through the uveoscleral pathway resulting in lowering the intraocular pressure. It also may act to improve blood flow to the ocular nerve. Systemic Alpha-1 adrenergic receptor antagonists have been implicated in Intraoperative Floppy Iris Syndrome (IFIS). Bunazosin potentially could have the same effect but there has been no research to substantiate this as a risk for cataract surgery.
This article is issued from Wikipedia - version of the 4/2/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.