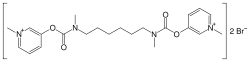
Distigmine
|
Distigmine bromide | |
| Clinical data | |
|---|---|
| Routes of administration | oral, i.m. |
| ATC code | N07AA03 (WHO) |
| Pharmacokinetic data | |
| Bioavailability | 4.65 % [1] |
| Biological half-life | 65 h [1] |
| Excretion | renal [1] |
| Identifiers | |
| |
| CAS Number | 15876-67-2 |
| PubChem (CID) | 27522 |
| ChemSpider |
25613 |
| UNII |
T940307O7B |
| KEGG | D01228 |
| ChEMBL |
CHEMBL1098285 |
| ECHA InfoCard | 100.036.360 |
| Chemical and physical data | |
| Formula | C22H32Br2N4O4 |
| Molar mass | 576.322 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| (verify) | |
Distigmine (as distigmine bromide) is a parasympathomimetic. Distigmine is similar to pyridostigmine and neostigmine but has a longer duration of action. It is available as tablets on prescription only. Distigmine has a greater risk of causing cholinergic crisis because of accumulation of the drug being more likely than with neostigmine or pyridostigmine and so distigmine is rarely used as a treatment for myasthenia gravis, unlike pyridostigmine and neostigmine.
References
This article is issued from Wikipedia - version of the 4/5/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.