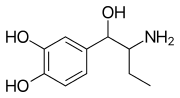
Ethylnorepinephrine
 | |
| Clinical data | |
|---|---|
| ATC code | None |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| Synonyms | β,3,4-trihydroxy-N-ethyl-2-phenylethylamine |
| CAS Number |
536-24-3 |
| PubChem (CID) | 18538 |
| ChemSpider |
17508 |
| UNII |
M6AY4VCZ0A |
| ChEMBL |
CHEMBL31159 |
| Chemical and physical data | |
| Formula | C10H15NO3 |
| Molar mass | 197.23 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Ethylnorepinephrine (Etanor, Bronkephrine, Butanefrine) is a sympathomimetic and bronchodilator related to norepinephrine.[1][2][3] It activates both α and β adrenergic receptors.[4]
See also
References
- ↑ David J. Triggle (1996). Dictionary of Pharmacological Agents. Boca Raton: Chapman & Hall/CRC. ISBN 0-412-46630-9.
- ↑ KORNEL L (1958). "A case of calcified ventricular aneurysm with progressive heart block; observations on the effect of ethylnorepinephrine". Cardiologia. 32 (2): 101–9. doi:10.1159/000165806. PMID 13500349.
- ↑ CHRISTENSEN JM, VALASEK FE, TAINTER ML (June 1958). "Ethylnorepinephrine; a unique bronchodilator". American Practitioner and Digest of Treatment. 9 (6): 916–21. PMID 13533786.
- ↑ Turner, Robert A. (1965). "12. Sympatholytic Agents. VI. The Two Kinds of Receptors". Screening Methods in Pharmacology. 111 Fifth Avenue, New York, New York 10003: Academic Press Inc. p. 150. ISBN 1483255913.
| Phenethylamines |
|
|---|---|
| Amphetamines |
|
| Phentermines |
|
| Cathinones |
|
| Phenylisobutylamines | |
| Phenylalkylpyrrolidines | |
| Catecholamines (and close relatives) |
|
| Miscellaneous |
|
Stimulants (category) | |
|---|---|
| Adamantanes |
|
| Adenosine antagonists | |
| Alkylamines | |
| Ampakines | |
| Arylcyclohexylamines | |
| Benzazepines | |
| Cholinergics |
|
| Convulsants | |
| Eugeroics | |
| Oxazolines | |
| Phenethylamines |
|
| Phenylmorpholines | |
| Piperazines | |
| Piperidines |
|
| Pyrrolidines | |
| Racetams | |
| Tropanes |
|
| Tryptamines | |
| Others |
|
This article is issued from Wikipedia - version of the 10/17/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.