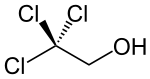
2,2,2-Trichloroethanol
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
2,2,2-Trichloroethanol | |||
| Identifiers | |||
| 115-20-8 | |||
| 3D model (Jmol) | Interactive image | ||
| ChEBI | CHEBI:28094 | ||
| ChEMBL | ChEMBL1171 | ||
| ChemSpider | 7961 | ||
| ECHA InfoCard | 100.003.701 | ||
| 2293 | |||
| KEGG | C07490 | ||
| UNII | AW835AJ62N | ||
| |||
| |||
| Properties | |||
| C2H3Cl3O | |||
| Molar mass | 149.40 g/mol | ||
| Density | 1.55 g/cm3 | ||
| Melting point | 17.8 °C (64.0 °F; 290.9 K) | ||
| Boiling point | 151 °C (304 °F; 424 K) | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
2,2,2-Trichloroethanol is an organic compound related to ethanol, except the hydrogen atoms at position 2 are replaced with chlorine atoms. In humans, its pharmacological effects are similar to those of its prodrugs, chloral hydrate and chlorobutanol. It has, historically, been used as a sedative hypnotic.[1] The hypnotic drug triclofos (2,2,2-trichloroethyl phosphate) is metabolized in vivo to 2,2,2-trichloroethanol. Chronic exposure may result in kidney and liver damage.[2]
See also
References
- ↑ The Merck Index, 13th Edition.
- ↑ S. Budavari; M. O'Neil; Ann Smith; P. Heckelman; J. Obenchain (15 March 1996). The Merck Index (12th print ed.). Taylor & Francis. ISBN 978-0-911910-12-4.
| Alcohols | |
|---|---|
| Barbiturates |
|
| Benzodiazepines |
|
| Carbamates | |
| Flavonoids | |
| Imidazoles | |
| Kava constituents |
|
| Monoureides |
|
| Neuroactive steroids |
|
| Nonbenzodiazepines |
|
| Phenols | |
| Piperidinediones | |
| Pyrazolopyridines | |
| Quinazolinones | |
| Volatiles/gases |
|
| Others/unsorted |
|
See also: GABAergics | |
| Receptor (ligands) |
| ||||||
|---|---|---|---|---|---|---|---|
| Transporter (blockers) |
| ||||||
| Others |
| ||||||
See also: GABAergics • GHBergics • Glutamatergics | |||||||
This article is issued from Wikipedia - version of the 9/14/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.