Febarbamate
 | |
| Clinical data | |
|---|---|
| ATC code | M03BA05 (WHO) |
| Identifiers | |
| |
| Synonyms | MS-543 |
| CAS Number |
13246-02-1 |
| PubChem (CID) | 25803 |
| ChemSpider |
24039 |
| UNII |
5Z48ONN38P |
| KEGG |
D07275 |
| ChEMBL |
CHEMBL2104283 |
| ECHA InfoCard | 100.032.919 |
| Chemical and physical data | |
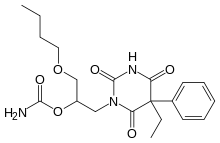
| Formula | C20H27N3O6 |
| Molar mass | 405.445 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Febarbamate (INN; Solium, Tymium), also known as phenobamate, is an anxiolytic and tranquilizer of the barbiturate and carbamate families which is used in Europe by itself and as part of a combination drug formulation called tetrabamate.[1][2][3][4]
See also
References
- ↑ World Health Organization (2004). "The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substance" (PDF).
- ↑ Index nominum 2000: international drug directory. Taylor & Francis US. 2000. p. 427. ISBN 978-3-88763-075-1. Retrieved 26 November 2011.
- ↑ Gentili E (March 1972). "[Therapeutic effects of a new psycholeptic agent (febarbamate, Solium) in pediatrics]". Minerva Medica (in Italian). 63 (18): 1058–60. PMID 5016064.
- ↑ Morton I, Hall JM (1999). Concise dictionary of pharmacological agents: properties and synonyms. Springer. p. 118. ISBN 978-0-7514-0499-9. Retrieved 26 November 2011.
Skeletal muscle relaxants (M03) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peripherally acting (primarily antinicotinic, NMJ block) |
| ||||||||||||||||
| Centrally acting | |||||||||||||||||
| Directly acting | |||||||||||||||||
| Alcohols | |
|---|---|
| Barbiturates |
|
| Benzodiazepines |
|
| Carbamates | |
| Flavonoids | |
| Imidazoles | |
| Kava constituents |
|
| Monoureides |
|
| Neuroactive steroids |
|
| Nonbenzodiazepines |
|
| Phenols | |
| Piperidinediones | |
| Pyrazolopyridines | |
| Quinazolinones | |
| Volatiles/gases |
|
| Others/unsorted |
|
See also: GABAergics | |
This article is issued from Wikipedia - version of the 10/11/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.