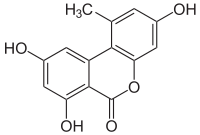
Alternariol
 | |
| Names | |
|---|---|
| IUPAC name
3,7,9-Trihydroxy-1-methyl-6H-benzo[c]chromen-6-one | |
| Identifiers | |
| 641-38-3 | |
| 3D model (Jmol) | Interactive image Interactive image |
| ChEBI | CHEBI:64983 |
| ChEMBL | ChEMBL519982 |
| ChemSpider | 4514301 |
| ECHA InfoCard | 100.164.145 |
| KEGG | C16838 |
| PubChem | 5359485 |
| UNII | KN9L4260JW |
| |
| |
| Properties | |
| C14H10O5 | |
| Molar mass | 258.23 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Alternariol is a toxic metabolite of Alternaria fungi.[1] It is an important contaminant in cereals and fruits.[2] Alternariol exhibits antifungal and phytotoxic activity. It is reported to inhibit cholinesterase enzymes.[3] It is also a mycoestrogen.
References
- ↑ Davis VM, Stack ME (1 October 1994). "Evaluation of alternariol and alternariol methyl ether for mutagenic activity in Salmonella typhimurium". Appl. Environ. Microbiol. 60 (10): 3901–2. PMC 201908
 . PMID 7986060.
. PMID 7986060. - ↑ Brugger EM, Wagner J, Schumacher DM, et al. (2006). "Mutagenicity of the mycotoxin alternariol in cultured mammalian cells". Toxicol. Lett. 164 (3): 221–30. doi:10.1016/j.toxlet.2006.01.001. PMID 16464542.
- ↑ Alternariol product page from Fermentek
| ER |
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| GPER |
| ||||||||||
See also: Androgenics • Glucocorticoidics • Mineralocorticoidics • Progestogenics • Steroid hormone metabolism modulators | |||||||||||
This article is issued from Wikipedia - version of the 7/15/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.