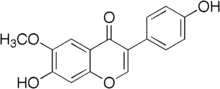
Glycitein
 | |
 | |
| Names | |
|---|---|
| IUPAC name
7-Hydroxy-3-(4-hydroxyphenyl)-6-methoxy-4-chromenone | |
| Other names
4',7-Dihydroxy-6-methoxyisoflavone | |
| Identifiers | |
| 40957-83-3 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:34778 |
| ChEMBL | ChEMBL513024 |
| ChemSpider | 4476508 |
| PubChem | 5317750 |
| |
| |
| Properties | |
| C16H12O5 | |
| Molar mass | 284.26 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Glycitein is an O-methylated isoflavone which accounts for 5-10% of the total isoflavones in soy food products. Glycitein is a phytoestrogen with weak estrogenic activity, comparable to that of the other soy isoflavones.[1]
References
- ↑ Song TT, Hendrich S, Murphy PA (1999). "Estrogenic activity of glycitein, a soy isoflavone". J. Agric. Food Chem. 47 (4): 1607–1610. doi:10.1021/jf981054j. PMID 10564025.
This article is issued from Wikipedia - version of the 5/31/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.