Potassium sulfite
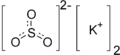 | |
| Names | |
|---|---|
| IUPAC name
Potassium sulfite | |
| Other names
E225 | |
| Identifiers | |
| 10117-38-1 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 23332 |
| ECHA InfoCard | 100.030.279 |
| E number | E225 (preservatives) |
| PubChem | 24958 |
| UNII | 015KZC652E |
| |
| |
| Properties | |
| K2SO3 | |
| Molar mass | 158.26 g/mol |
| Appearance | white solid |
| soluble | |
| Acidity (pKa) | 8 |
| Hazards | |
| Flash point | Non-flammable |
| Related compounds | |
| Other anions |
Potassium sulfate Potassium selenite |
| Other cations |
Sodium sulfite |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Potassium sulfite (K2SO3) is a chemical compound which is the salt of potassium cation and sulfite anion. As a food additive it is used as a preservative under the E number E225 (INS number 225). It is approved for use in Australia and New Zealand[1] and is not approved in the EU.[2]
References
- ↑ Australia New Zealand Food Standards Code"Standard 1.2.4 - Labelling of ingredients". Retrieved 2011-10-27.
- ↑ UK Food Standards Agency: "Current EU approved additives and their E Numbers". Retrieved 2011-10-27.
This article is issued from Wikipedia - version of the 11/14/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.