Fluticasone
Not to be confused with fluconazole.
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Monograph |
| Pregnancy category |
|
| Routes of administration | Intranasal, inhaled, topical cream or ointment |
| ATC code | D07AC17 (WHO) R01AD08 (WHO) R03BA05 (WHO) |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 0.51% (Intranasal) |
| Protein binding | 91% |
| Metabolism |
Intranasal Hepatic (CYP3A4-mediated) |
| Biological half-life | 10 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number |
90566-53-3 |
| PubChem (CID) | 5311101 |
| IUPHAR/BPS | 6699 |
| DrugBank |
DB00588 |
| ChemSpider |
4470631 |
| UNII |
CUT2W21N7U |
| KEGG |
D07981 |
| ChEBI |
CHEBI:5134 |
| ChEMBL |
CHEMBL1201396 |
| Chemical and physical data | |
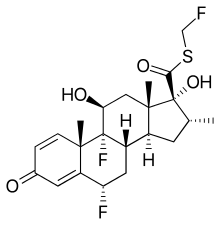
| Formula | C22H27F3O4S |
| Molar mass | 444.508 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Fluticasone is a synthetic glucocorticoid.[1] Both the furoate and propanoate esters, fluticasone furoate and fluticasone propionate, are used as topical anti-inflammatories[2] and inhaled corticosteroids.
References
- ↑ Briggs, Gerald G.; Freeman, Roger K.; Yaffe, Sumner J. (2012), Drugs in Pregnancy and Lactation: A Reference Guide to Fetal and Neonatal Risk, Lippincott Williams & Wilkins, p. 600, ISBN 1451153597.
- ↑ Spratto, George R.; Woods, Adrienne L. (2012), Delmar Nurse's Drug Handbook 2012, Cengage Learning, p. 748, ISBN 1111310653.
This article is issued from Wikipedia - version of the 11/5/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.