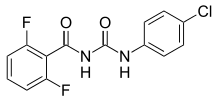
Diflubenzuron
 | |
 | |
| Names | |
|---|---|
| IUPAC name
N-[(4-Chlorophenyl)carbamoyl]-2,6-difluorobenzamide | |
| Other names
Dimilin | |
| Identifiers | |
| 35367-38-5 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:34703 |
| ChEMBL | ChEMBL49338 |
| ChemSpider | 34065 |
| ECHA InfoCard | 100.047.740 |
| KEGG | C14427 |
| PubChem | 37123 |
| UNII | J76U6ZSI8D |
| |
| |
| Properties[1] | |
| C14H9ClF2N2O2 | |
| Molar mass | 310.68 g·mol−1 |
| 0.08 mg/L | |
| Solubility in other solvents | DMSO: 12 g/100 g Acetone 0.615 g/100 g Methanol: 0.09 g/100 g |
| Pharmacology | |
| QP53BC02 (WHO) | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Diflubenzuron is a benzoylurea-type insecticide of the benzamide class. It is used in forest management and on field crops[2] to selectively control insect pests, particularly forest tent caterpillar moths, boll weevils, gypsy moths, and other types of moths.[1] The mechanism of action of diflubenzuron involves inhibiting the production of chitin which is used by an insect to build its exoskeleton.[1]
Environmental Toxicity
Diflubenzuron has been evaluated by the United States Environmental Protection Agency (EPA), and it is classified as non-carcinogenic. While 4-Chloroaniline, a metabolite of diflubenzuron, has been classified as a Carcinogen, it is not present in diflubenzuron. 4-Chloroanaline is produced after diflubenzuron has been ingested, and the body changes a small fraction of it to the metabolite. Diflubenzuron has been tested, and the small amount converted to 4-chloroanilnine after ingestion does not cause cancer.[3]
References
- 1 2 3 Diflubenzuron Pesticide Information Profile, Extension Toxicology Network
- ↑ Johnson, Douglas (2016). "Insecticide Recommendations for Soybeans - 2016" (PDF). Cooperative Extension Service. University of Kentucky: College of Agriculture, Food and Environment. Retrieved 16 February 2016.
- ↑ "Reregistration Eligibility Decision Diflubenzuron" (PDF). Environmental Protection Agency. Retrieved 16 November 2016.