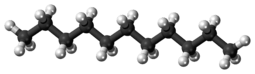
Undecane
| | |
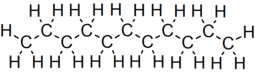 | |
 | |
| Names | |
|---|---|
| IUPAC name
Undecane[1] | |
| Identifiers | |
| 1120-21-4 | |
| 3D model (Jmol) | Interactive image |
| 1697099 | |
| ChEBI | CHEBI:46342 |
| ChEMBL | ChEMBL132474 |
| ChemSpider | 13619 |
| ECHA InfoCard | 100.013.001 |
| EC Number | 214-300-6 |
| MeSH | undecane |
| PubChem | 14257 |
| RTECS number | YQ1525000 |
| UNII | JV0QT00NUE |
| UN number | 2330 |
| |
| |
| Properties | |
| C11H24 | |
| Molar mass | 156.31 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | Gasoline-like to Odorless |
| Density | 740 mg mL−1 |
| Melting point | −26.6 to −25.0 °C; −15.8 to −12.9 °F; 246.6 to 248.2 K |
| Boiling point | 193 to 197 °C; 379 to 386 °F; 466 to 470 K |
| log P | 6.312 |
| Vapor pressure | 55 Pa (at 25 °C)[2] |
| Henry's law constant (kH) |
5.4 nmol Pa−1 kg−1 |
| Refractive index (nD) |
1.417 |
| Thermochemistry | |
| 345.05 J K−1 mol−1 | |
| Std molar entropy (S |
458.15 J K−1 mol−1 |
| Std enthalpy of formation (ΔfH |
−329.8–−324.6 kJ mol−1 |
| Std enthalpy of combustion (ΔcH |
−7.4339–−7.4287 MJ mol−1 |
| Hazards | |
| GHS pictograms |  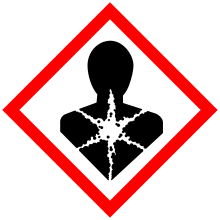 |
| GHS signal word | DANGER |
| H304, H315, H319, H331, H335 | |
| P261, P301+310, P305+351+338, P311, P331 | |
| EU classification (DSD) |
|
| R-phrases | R36/37/38, R65 |
| S-phrases | S26, S36 |
| Flash point | 62.0 °C (143.6 °F; 335.1 K) |
| Related compounds | |
| Related alkanes |
|
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Undecane (also known as hendecane) is a liquid alkane hydrocarbon with the chemical formula CH3(CH2)9CH3. It is used as a mild sex attractant for various types of moths and cockroaches, and an alert signal for a variety of ants.[3] It has 159 isomers.
Undecane may also be used as an internal standard in gas chromatography when working with other hydrocarbons. Since the boiling point of undecane (196 °C) is well known, it may be used as a comparison for retention times in a gas chromatograph for molecules whose structure has been freshly elucidated. For example, if one is working with a 50 m crosslinked methyl silicone capillary column with an oven temperature increasing slowly, beginning around 60 °C, an 11-carbon molecule like undecane may be used as an internal standard to be compared with the retention times of other 10-, 11-, or 12- carbon molecules, depending on their structures.
See also
References
- ↑ "undecane - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 16 September 2004. Identification and Related Records. Retrieved 5 January 2012.
- ↑ Yaws, Carl L. (1999). Chemical Properties Handbook. New York: McGraw-Hill. pp. 159–179. ISBN 0-07-073401-1.
- ↑ Hölldobler B, Wilson EO (1990). The Ants. Harvard University Press. ISBN 0-674-04075-9, p. 287
External links
- Undecane at Dr. Duke's Phytochemical and Ethnobotanical Databases