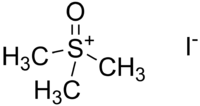
Trimethylsulfoxonium iodide
 | |
 | |
| Names | |
|---|---|
| IUPAC name
S,S,S-Trimethylsulfoxonium iodide | |
| Other names
Trimethylsulphoxonium iodide; Trimethyloxosulfonium iodide | |
| Identifiers | |
| 1774-47-6 | |
| 3D model (Jmol) | Interactive image |
| ChEMBL | ChEMBL283149 |
| ECHA InfoCard | 100.015.641 |
| |
| Properties | |
| C3H9IOS | |
| Molar mass | 220.07 g·mol−1 |
| Melting point | 208 to 212 °C (406 to 414 °F; 481 to 485 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Trimethylsulfoxonium iodide is a sulfoxonium salt. It is used to generate dimethyloxosulfonium methylide by reaction with sodium hydride.[1] The latter compound is used as a methylene-transfer reagent, and is used to prepare epoxides.
This compound is commercially available. It may be prepared by the reaction of dimethyl sulfoxide and iodomethane:[2]
- (CH3)2SO + CH3I → (CH3)3SO+I−
References
- ↑ E. J. Corey1 and Michael Chaykovsky2. "Methylenecyclohexane Oxide". Org. Synth.; Coll. Vol., 5, p. 755
- ↑ Lampman, Gary M.; Koops, Roger W.; Olden, Caroline C. (1985). "Phosphorus and sulfur ylide formation: Preparation of 1-benzoyl-2-phenylcyclopropane and 1,4-diphenyl-1,3-butadiene by phase transfer catalysis". J. Chem. Ed. 62 (3): 267. doi:10.1021/ed062p267.
This article is issued from Wikipedia - version of the 7/5/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.