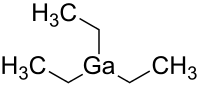
Triethylgallium
 | |
| Names | |
|---|---|
| IUPAC name
triethylgallane | |
| Systematic IUPAC name
triethylgallium | |
| Identifiers | |
| 1115-99-7 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 59583 |
| ECHA InfoCard | 100.012.939 |
| PubChem | 66198 |
| |
| Properties | |
| C6H15Ga | |
| Molar mass | 156.9 g/mol |
| Appearance | clear colourless liquid |
| Melting point | −82.3 °C (−116.1 °F; 190.8 K) |
| Boiling point | 143 °C (289 °F; 416 K) |
| Reacts[1] | |
| Hazards | |
| Main hazards | pyrophoric |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Triethylgallium, Ga(C2H5)3, or TEGa, is a metalorganic source of gallium for metalorganic vapour phase epitaxy (MOVPE) of compound semiconductors.
Properties
TEGa is a clear, colorless, pyrophoric liquid[2] and should be handled with caution.
Applications
TEGa can be a useful alternative to trimethylgallium in the metalorganic vapour phase epitaxy of compound semiconductors because films grown using TEGa have been shown to have a lower carbon impurity concentration.[3]
References
- ↑ amdg.ece.gatech.edu/msds/mo/teg_epichem.pdf
- ↑ Shenaikhatkhate, D; Goyette, R; Dicarlojr, R; Dripps, G (2004). "Environment, health and safety issues for sources used in MOVPE growth of compound semiconductors". Journal of Crystal Growth. 272: 816. Bibcode:2004JCrGr.272..816S. doi:10.1016/j.jcrysgro.2004.09.007.
- ↑ Saxler, A; Walker, D; Kung, P; Zhang, X; Razeghi, M; Solomon, J; Mitchel, W; Vydyanath, H (1997). "Comparison of trimethylgallium and triethylgallium for the growth of GaN". Applied Physics Letters. 71: 3272. Bibcode:1997ApPhL..71.3272S. doi:10.1063/1.120310.
This article is issued from Wikipedia - version of the 2/2/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.