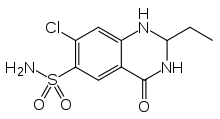
Quinethazone
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Routes of administration | Oral |
| ATC code | C03BA02 (WHO) |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
73-49-4 |
| PubChem (CID) | 6307 |
| IUPHAR/BPS | 7289 |
| DrugBank |
DB01325 |
| ChemSpider |
6068 |
| UNII |
455E0S048W |
| KEGG |
D00461 |
| ChEMBL |
CHEMBL1532 |
| ECHA InfoCard | 100.000.729 |
| Chemical and physical data | |
| Formula | C10H12ClN3O3S |
| Molar mass | 289.739 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| (verify) | |
Quinethazone (INN, brand name Hydromox) is a thiazide-like diuretic used to treat hypertension. Common side effects include dizziness, dry mouth, nausea, and low potassium levels.
References
This article is issued from Wikipedia - version of the 4/2/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.