Oxygen difluoride
 | |
 | |
| Names | |
|---|---|
| Other names
oxygen fluoride hypofluorous anhydride | |
| Identifiers | |
| 7783-41-7 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:30494 |
| ChemSpider | 22953 |
| ECHA InfoCard | 100.029.087 |
| EC Number | 231-996-7 |
| PubChem | 24547 |
| RTECS number | RS2100000 |
| |
| |
| Properties | |
| OF2 | |
| Molar mass | 53.9962 g/mol |
| Appearance | colorless gas, pale yellow liquid when condensed |
| Odor | peculiar, foul |
| Density | 1.90 g/cm3 (-224° C, liquid), 1.719 g/cm3 (-183° C, liquid), 1.521 g/cm3 (liquid at −145 °C), 1.88 g/l (gas at room temperature) |
| Melting point | −223.8 °C (−370.8 °F; 49.3 K) |
| Boiling point | −144.75 °C (−228.55 °F; 128.40 K) |
| hydrolyzes[1] | |
| Vapor pressure | >1 atm (20°C)[2] |
| Thermochemistry | |
| 43.3 J/mol K | |
| Std molar entropy (S |
246.98 J/mol K |
| Std enthalpy of formation (ΔfH |
24.5 kJ mol−1 |
| Gibbs free energy (ΔfG˚) |
42.5 kJ/mol |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
| LC50 (median concentration) |
2.6 ppm (rat, 1 hr) 1.5 ppm (mouse, 1 hr) 26 ppm (dog, 1 hr) 16 ppm (monkey, 1 hr)[3] |
| US health exposure limits (NIOSH): | |
| PEL (Permissible) |
TWA 0.05 ppm (0.1 mg/m3)[2] |
| REL (Recommended) |
C 0.05 ppm (0.1 mg/m3)[2] |
| IDLH (Immediate danger) |
0.5 ppm[2] |
| Related compounds | |
| Related compounds |
HFO O2F2 NHF2 NF3 SCl2 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
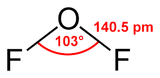
Oxygen difluoride is the chemical compound with the formula OF2. As predicted by VSEPR theory, the molecule adopts a "bent" molecular geometry similar to that of water, but it has very different properties, being a strong oxidizer.
Preparation
Oxygen difluoride was first reported in 1929; it was obtained by the electrolysis of molten potassium fluoride and hydrofluoric acid containing small quantities of water.[4][5] The modern preparation entails the reaction of fluorine with a dilute aqueous solution of sodium hydroxide, with sodium fluoride as a side-product:
- 2 F2 + 2 NaOH → OF2 + 2 NaF + H2O
Reactions
Its powerful oxidizing properties are suggested by the oxidation number of +2 for the oxygen atom instead of its normal -2. Above 200 °C, OF2 decomposes to oxygen and fluorine via a radical mechanism.
OF2 reacts with many metals to yield oxides and fluorides. Nonmetals also react: phosphorus reacts with OF2 to form PF5 and POF3; sulfur gives SO2 and SF4; and unusually for a noble gas, xenon reacts, at elevated temperatures, yielding XeF4 and xenon oxyfluorides.
Oxygen difluoride reacts very slowly with water to form hydrofluoric acid:
- OF2 (aq) + H2O (l) → 2 HF (aq) + O2 (g)
Oxygen difluoride oxidizes sulfur dioxide to sulfur trioxide:
- OF2 + SO2 → SO3 + F2
However, in the presence of UV radiation the products are sulfuryl fluoride, SO
2F
2, and pyrosulfuryl fluoride, S
2O
5F
2:
- OF2 + 2 SO2 → S
2O
5F
2
Popular culture
In Robert L. Forward's science fiction novel Camelot 30K, oxygen difluoride was used as a biochemical solvent by fictional life forms living in the solar system's Kuiper belt.
Safety
OF2 is a dangerous chemical, as is the case for any strongly oxidizing gas.
References
- ↑ http://www.chemyq.com/En/xz/xz1/2818mqnrv.htm
- 1 2 3 4 "NIOSH Pocket Guide to Chemical Hazards #0475". National Institute for Occupational Safety and Health (NIOSH).
- ↑ "Oxygen difluoride". Immediately Dangerous to Life and Health. National Institute for Occupational Safety and Health (NIOSH).
- ↑ Lebeau, P.; Damiens, A. (1929). "Sur un nouveau mode de préparation du fluorure d'oxygène" [A new method of preparation of oxygen fluoride]. Comptes rendus hebdomadaires des séances de l’Académie des sciences (in French). 188: 1253–1255. Retrieved February 21, 2013.
- ↑ Lebeau, P.; Damiens, A. (1927). "Sur l'existence d'un composé oxygéné du fluor" [The existence of an oxygen compound of fluorine]. Comptes rendus hebdomadaires des séances de l’Académie des sciences (in French). 185: 652–654. Retrieved February 21, 2013.
External links
- National Pollutant Inventory - Fluoride and compounds fact sheet
- WebBook page for OF2
- CDC - NIOSH Pocket Guide to Chemical Hazards