New fuchsine
 | |
| Names | |
|---|---|
| Other names
new fuchsin, Magenta III, Basic Violet 2, C.I. 42520 | |
| Identifiers | |
| 3248-91-7 | |
| ChEBI | CHEBI:87671 |
| ECHA InfoCard | 100.019.847 |
| Properties | |
| C22H24N3Cl | |
| Appearance | dark red solid |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
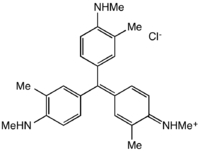
New fuchsine, (from German "fuchs", fox) is an organic compound with the formula [(CH3N(H)CH3C6H3)3C]Cl. It is a magenta-colored solid that is used as a dye. It is one of the four components of basic fuchsine, and one of the two that are available as single dyes.[1] The other is pararosaniline.
It is prepared by condensation of N-methyltoluidine with xylidene in the presence of hydrochloric acid.
Use as dye and stain
It is used to dye polyacrylonitrile, paper, and leather.[2]
New fuchsine can be used for staining acid fast organism, e.g. by Ziehl-Neelsen stain, and for making Schiff's reagent. As a primary amine, the dye can be diazotized in the laboratory, and the resulting diazonium salt used as a trapping agent in enzyme histochemistry.[3]
See also
External links
References
- ↑ Horobin RW, Kiernan JA (2002) Conn's Biological Stains, 10th ed. Oxford: BIOS.
- ↑ Thomas Gessner and Udo Mayer "Triarylmethane and Diarylmethane Dyes" in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim.doi:10.1002/14356007.a27_179
- ↑ Lojda Z, Gossrau R, Schiebler TH (1979) Enzyme Histochemistry. A Laboratory Manual. Berlin: Springer-Verlag.
This article is issued from Wikipedia - version of the 8/26/2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.