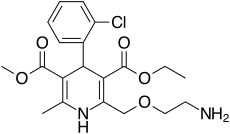
Amlodipine/benazepril
 | |
 | |
| Combination of | |
|---|---|
| Amlodipine | Calcium channel blocker |
| Benazepril | ACE inhibitor |
| Clinical data | |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | None |
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number |
357437-90-2 |
| PubChem (CID) | 5746247 |
| ChemSpider |
4676979 |
| | |
Amlodipine/benazepril, marketed in the U.S. as Lotrel by Novartis and manufactured as a generic drug by Teva and Sandoz, is an antihypertensive medication which combines a calcium channel blocker (amlodipine besilate) with an angiotensin converting enzyme inhibitor (benazepril).[1] This drug, like similar combinations, is prescribed when either agent alone is not sufficient to bring a person's blood pressure down to target range. As a combination agent, Lotrel shares the adverse reaction profile of both of its individual parts.[2][3]
See also
References
External links
This article is issued from Wikipedia - version of the 11/21/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.