Iodine monobromide
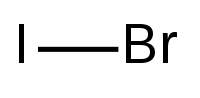 | |
 | |
| Names | |
|---|---|
| IUPAC name
Iodine monobromide | |
| Other names
Iodine bromide | |
| Identifiers | |
| 7789-33-5 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 74216 |
| ECHA InfoCard | 100.029.236 |
| PubChem | 82238 |
| |
| |
| Properties | |
| IBr | |
| Molar mass | 206.904 g/mol |
| Appearance | dark red solid |
| Melting point | 42 °C (108 °F; 315 K) |
| Boiling point | 116 °C (241 °F; 389 K) |
| Related compounds | |
| Other anions |
iodine monochloride, iodine monofluoride |
| Related interhalogen compounds |
Iodine monochloride Iodine monofluoride Bromine monochloride |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Iodine monobromide is an interhalogen compound with the chemical symbol IBr. It is a dark red solid that melts near room temperature. Like iodine monochloride, IBr is used in some types of iodometry. It serves as a source of I+.
Synthesis
Iodine monobromide is formed when iodine and bromine are combined:[1]
- I2 + Br2 → 2 IBr
References
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 0-08-037941-9.
This article is issued from Wikipedia - version of the 6/21/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.