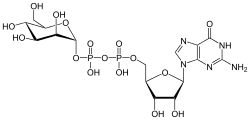
Guanosine diphosphate mannose
 | |
| Names | |
|---|---|
| IUPAC name
[(2R,3S,4R,5R)-5-(2-amino-6-oxo-1,6-dihydro-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl]methyl (2R,3S,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl dihydrogen diphosphate | |
| Identifiers | |
| 3123-67-9 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:15820 |
| ChemSpider | 17372 |
| MeSH | Guanosine+Diphosphate+Mannose |
| PubChem | 18396 |
| |
| |
| Properties | |
| C16H25N5O16P2 | |
| Molar mass | 605.341 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Guanosine diphosphate mannose or GDP-mannose is a nucleotide sugar that is a substrate for glycosyltransferase reactions in metabolism. This compound is a substrate for enzymes called mannosyltransferases.
Biosynthesis
GDP-mannose is produced from GTP and mannose-6-phosphate by the enzyme mannose-1-phosphate guanylyltransferase.[1]
References
- ↑ Samuel G, Reeves P (2003). "Biosynthesis of O-antigens: genes and pathways involved in nucleotide sugar precursor synthesis and O-antigen assembly". Carbohydr. Res. 338 (23): 2503–19. doi:10.1016/j.carres.2003.07.009. PMID 14670712.
See also
This article is issued from Wikipedia - version of the 5/20/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.