Dehydroacetic acid
 | |
| Names | |
|---|---|
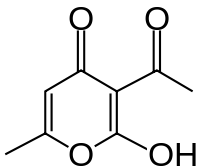
| Preferred IUPAC name
3-Acetyl-2-hydroxy-6-methyl-4H-pyran-4-one | |
| Other names
Biocide 470F Methylacetopyronone | |
| Identifiers | |
| 520-45-6 | |
| 3D model (Jmol) | Interactive image |
| Abbreviations | DHAA |
| ChEMBL | ChEMBL284127 |
| ChemSpider | 10177 |
| ECHA InfoCard | 100.007.541 |
| EC Number | 208-293-9 |
| E number | E265 (preservatives) |
| MeSH | dehydroacetic+acid |
| PubChem | 10623 |
| UNII | 2KAG279R6R |
| |
| |
| Properties | |
| C8H8O4 | |
| Molar mass | 168.15 g·mol−1 |
| Appearance | White crystals |
| Melting point | 109 °C; 228 °F; 382 K |
| Boiling point | 270 °C; 518 °F; 543 K |
| Hazards | |
| EU classification (DSD) |
|
| R-phrases | R22 |
| S-phrases | (S2) |
| NFPA 704 | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Dehydroacetic acid is an organic compound. The compound is classified as a pyrone derivative and is used mostly as a fungicide and bactericide. The sodium salt, sodium dehydroacetate, is often used in place of dehydroacetic acid because of its greater solubility in water. It is prepared by the base-catalysed dimerization of diketene.[2]
Applications
Industrially, it is also used as a plasticizer in synthetic resins.[1] It is used to reduce pickle bloating as a preservative for squash and strawberries.[3] Also used in antienzyme toothpastes. When used as a food additive, dehydroacetic acid is referred to using the International Numbering System for Food Additives or E number 265.
References
- 1 2 Merck Index, 11th Edition, 2855
- ↑ Raimund Miller, Claudio Abaecherli, Adel Said, Barry Jackson "Ketenes" in Ullmann's Encyclopedia of Industrial Chemistry, 2001, Wiley-VCH, Weinheim. doi: 10.1002/14356007.a15_063
- ↑ Handbook of Biocide and Preservative Use, Harold William Rossmoore, p. 341 ISBN 0-7514-0212-5
This article is issued from Wikipedia - version of the 7/15/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.
