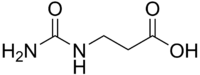
3-Ureidopropionic acid
 | |
| Names | |
|---|---|
| Preferred IUPAC name
3-Ureidopropanoic acid | |
| Systematic IUPAC name
3-(Carbamoylamino)propanoic acid[1] | |
| Identifiers | |
| 462-88-4 | |
| 3D model (Jmol) | Interactive image Interactive image |
| 3DMet | B00472 |
| 1705263 | |
| ChEBI | CHEBI:18261 |
| ChEMBL | ChEMBL20962 |
| ChemSpider | 109 |
| 675230 | |
| KEGG | C02642 |
| MeSH | N-carbamoyl-beta-alanine |
| PubChem | 111 |
| |
| |
| Properties | |
| C4H8N2O3 | |
| Molar mass | 132.12 g·mol−1 |
| Appearance | White crystals |
| log P | −1.23 |
| Acidity (pKa) | 4.408 |
| Basicity (pKb) | 9.589 |
| Hazards | |
| S-phrases | S22, S24/25 |
| Related compounds | |
| Related alkanoic acids |
|
| Related compounds |
|
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
3-Ureidopropionic acid, also called N-carbamoyl-beta-alanine, is an intermediate in the metabolism of uracil. It is a urea derivative of beta-alanine.
References
- ↑ "N-carbamoyl-beta-alanine - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 16 September 2004. Identification. Retrieved 28 June 2012.
This article is issued from Wikipedia - version of the 6/24/2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.