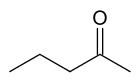
2-Pentanone
 | |
 | |
| Names | |
|---|---|
| IUPAC name
2-pentanone | |
| Other names
methyl propyl ketone 2-pentanone MPK | |
| Identifiers | |
| 107-87-9 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:16472 |
| ChEMBL | ChEMBL45345 |
| ChemSpider | 7607 |
| ECHA InfoCard | 100.003.208 |
| KEGG | C01949 |
| PubChem | 7895 |
| RTECS number | CY1400000 |
| UNII | I97392I10V |
| |
| |
| Properties | |
| C5H10O | |
| Molar mass | 86.13 g/mol |
| Appearance | Colorless liquid |
| Odor | resembling acetone |
| Density | 0.809 g/ml |
| Melting point | −78 °C (−108 °F; 195 K) |
| Boiling point | 102 °C (216 °F; 375 K) |
| 6% (20°C)[1] | |
| Vapor pressure | 3.6 kPa (20 °C) |
| Refractive index (nD) |
1.390 (20 °C) |
| Viscosity | 0.50 mPa·s (20 °C) |
| Hazards | |
| Flash point | 10 °C (50 °F) |
| Explosive limits | 1.5%-8.2%[1] |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) |
1600 mg/kg (rat, oral) 1600 mg/kg (mouse, oral)[2] |
| LCLo (lowest published) |
50,000 ppm (guinea pig, 50 min) 13,000 ppm (guinea pig, 5 hr)[2] |
| US health exposure limits (NIOSH): | |
| PEL (Permissible) |
TWA 200 ppm (700 mg/m3)[1] |
| REL (Recommended) |
TWA 150 ppm (530 mg/m3)[1] |
| IDLH (Immediate danger) |
1500 ppm[1] |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
2-Pentanone or methyl propyl ketone (MPK) is a ketone and solvent of minor importance. It is comparable to methyl ethyl ketone, but has a lower solvency and is more expensive.[3] It occurs naturally in Nicotiana tabacum (Tobacco).[4]
References
- 1 2 3 4 5 "NIOSH Pocket Guide to Chemical Hazards #0488". National Institute for Occupational Safety and Health (NIOSH).
- 1 2 "2-Pentanone". Immediately Dangerous to Life and Health. National Institute for Occupational Safety and Health (NIOSH).
- ↑ Dieter Stoye (2007), "Solvents", Ullmann's Encyclopedia of Industrial Chemistry (7th ed.), Wiley, pp. 55–56
- ↑ T. C. Tso (2007), "Tobacco", Ullmann's Encyclopedia of Industrial Chemistry (7th ed.), Wiley, p. 19
This article is issued from Wikipedia - version of the 2/4/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.