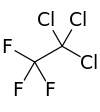
1,1,1-Trichloro-2,2,2-trifluoroethane
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
1,1,1-Trichloro-2,2,2-trifluoroethane | |||
| Other names
CFC-113a Freon 113a Arcton 63 Freon-FT 1,1,1-Trichloro-2,2,2-trifluoroethane 1,1,1-Trichlorotrifluoroethane 1,1,1-Trifluoro-2,2,2-trichloroethane 1,1,1-Trifluorotrichloroethane CF3CCl3 FC 113 FC133a Precision cleaning agent TF T-WD602 Trichlorotrifluoroethane FC 113a 2,2,2-Trichloro-1,1,1-trifluoro-ethane | |||
| Identifiers | |||
| 354-58-5 | |||
| 3D model (Jmol) | Interactive image | ||
| ECHA InfoCard | 100.005.968 | ||
| EC Number | 206-564-6 | ||
| |||
| |||
| Properties | |||
| C2Cl3F3 | |||
| Molar mass | 187.376 g/mol | ||
| Density | 1.579 g/mL[1][2] | ||
| Melting point | 13–14 °C (55–57 °F; 286–287 K) | ||
| Boiling point | 46 °C (115 °F; 319 K) | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Trichlorotrifluoroethane, also called 1,1,1-Trichloro-2,2,2-trifluoroethane or CFC-113a is a chlorofluorocarbon (CFC). It has the formula Cl3C-CF3.
Environmental effects
Ozone depletion
It is one of four man-made chemicals newly discovered in the atmosphere by a team at the University of East Anglia. But CFC-113a is the only known CFC whose abundance in the atmosphere is still growing. CFC-113a seems to have been accumulating unabated since 1960. Its source remains a mystery, but illegal manufacturing in China is suspected by some. Between 2010 and 2012, emissions of the gas jumped by 45 percent.[3][4]
See also
References
- ↑ "1,1,1-Trichlorotrifluoroethane". chemblink.com. Retrieved 10 March 2014.
- ↑ "Material Safety Data Sheet : 1,1,1-Trichlorotrifluoroethane". fishersci.com. Retrieved 10 March 2014.
- ↑ Laube, Johannes C.; Newland, Mike J.; Hogan, Christopher; Brenninkmeijer, Carl A. M.; Fraser, Paul J.; Martinerie, Patricia; Oram, David E.; Reeves, Claire E.; Röckmann, Thomas; Schwander, Jakob; Witrant, Emmanuel; Sturges, William T. (9 March 2014). "Newly detected ozone-depleting substances in the atmosphere". Nature Geoscience. doi:10.1038/ngeo2109. Retrieved 10 March 2014.
- ↑ McGrath, Matt. "Mysterious new man-made gases pose threat to ozone layer". BBC. Retrieved 10 March 2014.
This article is issued from Wikipedia - version of the 4/12/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.